Chast' I. FIZIKA ZVEZDNO' MATERII
Glava 1. Termodinamicheskie svoistva veshestva
Razdely
- 1. Ideal'nyi gaz s izlucheniem
- 2. Relyativistskii gaz s uchetom vyrozhdeniya
- 3. Uravnenie sostoyaniya pri nalichii yadernogo ravnovesiya i processov slabogo vzaimodeistviya
- 4. Veshestvo pri ochen' bol'shih plotnostyah, neitronizaciya, vzaimodeistvie chastic
Veshestvo bol'shinstva zvezd imeet vysokuyu temperaturu i sravnitel'no umerennuyu plotnost'. V etih usloviyah kineticheskaya energiya chastic mnogo bol'she energii vzaimodeistviya mezhdu nimi i model' nerelyativistskogo, nevyrozhdennogo ideal'nogo gaza okazyvaetsya horoshim priblizheniem k real'nosti. Termodinamicheskie svoistva veshestva planet, naprimer. Zemli, izucheny gorazdo huzhe. Temperatura ih pri toi zhe plotnosti znachitel'no nizhe i veshestvo nahoditsya v zhidkoi i tverdoi fazah, issledovanie kotoryh sopryazheno s sushestvennymi trudnostyami.
V nedrah zvezd veshestvo i izluchenie nahodyatsya v termodinamicheskom ravnovesii, kotoroe ustanavlivaetsya bystrymi processami stolknovenii chastic, poglosheniem i ispuskaniem fotonov. Izluchenie, naryadu s gazom, sozdaet davlenie, protivodeistvuyushee sile tyazhesti.
Veshestvo zvezd sostoit iz razlichnyh himicheskih elementov, osnovnymi iz kotoryh yavlyayutsya vodorod i gelii. Na Solnce, naprimer, oni sostavlyayut v summe bolee 98,5% plotnosti veshestva. Ostal'naya chast' massy Solnca sostoit iz smesi prakticheski vseh stabil'nyh izotopov tablicy Mendeleeva. V tabl. 1 ukazano soderzhanie naibolee obil'nyh elementov, nablyudaemyh na Solnce [5]. Pri izmenenii ot centra do poverhnosti zvezdy temperatury na tri-chetyre poryadka i plotnosti na ~ 10 poryadkov izmenyaetsya sostoyanie ionizacii veshestva.
V central'nyh oblastyah zvezd s
![]() vse atomy prakticheski polnost'yu ionizovany.
vse atomy prakticheski polnost'yu ionizovany.
Pust' ![]() - nomer himicheskogo elementa, kotoryi mozhet
nahodit'sya v razlichnyh sostoyaniyah ionizacii ot neitral'nogo (
- nomer himicheskogo elementa, kotoryi mozhet
nahodit'sya v razlichnyh sostoyaniyah ionizacii ot neitral'nogo (![]() )
do polnost'yu ionizovannogo (
)
do polnost'yu ionizovannogo (![]() ). Oboznachim cherez
). Oboznachim cherez ![]() energiyu svyazi
energiyu svyazi ![]() -kratno ionizovannogo iona elementa
-kratno ionizovannogo iona elementa ![]() ,
opredelyaemuyu tak, chto dlya polnost'yu ionizovannogo iona
,
opredelyaemuyu tak, chto dlya polnost'yu ionizovannogo iona ![]() = 0. Udel'naya energiya E (erg g-1), davlenie R
(din sm-2) i udel'naya entropiya S (erg g-1 K-1) dannoi smesi atomov, ionov i elektronov s izlucheniem
imeyut vid [145] .
= 0. Udel'naya energiya E (erg g-1), davlenie R
(din sm-2) i udel'naya entropiya S (erg g-1 K-1) dannoi smesi atomov, ionov i elektronov s izlucheniem
imeyut vid [145] .
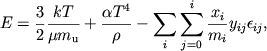
|
(1.1) |
|
|
(1.2) |
![$$
%\begin{displaymath}S=\frac{k}{\rho }\Sigma _{i}\Sigma _{j=0}^{i}n_{ij}\left\{ \frac{5}{2}+\ln\left[ \left( \frac{m_{i}kT}{2\pi \hbar ^{2}}\right) ^{3/2}\frac{g_{ij}}{n_{ij}}\right] \right\} +\frac{k}{\rho }n_{e}\left\{ \frac{5}{2}+\ln\left[ \left( \frac{m_{e}kT}{2\pi \hbar }\right) ^{3/2}\frac{2}{n_{e}}\right] \right\} +\frac{4}{3}\frac{aT^{3}}{\rho } \end{displaymath}
\eqalign{
S&={k\over\rho}\sum_i \sum_{j=0}^i n_{ij}\left\{ {5\over 2} \ln\left[
\left(m_i k T\over 2\pi \hbar^2\right)^{3/2}
{g_{ij}\over n_{ij}}\right]\right\}\cr
&\qquad+{k\over \rho} n_{\rm e} \left\{ {5\over 2}+\ln\left[
\left(m_{\rm e} k T\over 2\pi \hbar^2\right)^{3/2} {2\over n_{\rm e}} \right]\right\}
+{4\over 3} {a T^3\over \rho},\cr
}
$$](https://images.astronet.ru/pubd/2006/01/25/0001211125/tex/formula715.gif) |
(1.3) |
![]() - plotnost',
- plotnost',
![]() - temperatura,
- temperatura,
![]() - postoyannaya Bol'cmana,
- postoyannaya Bol'cmana,
![]() - postoyannaya Planka,
- postoyannaya Planka,
![]() - postoyannaya plotnosti izlucheniya,
- postoyannaya plotnosti izlucheniya,
![]() - skorost' sveta v vakuume,
- skorost' sveta v vakuume,
![]() - massovaya dolya elementa s atomnym nomerom i,
- massovaya dolya elementa s atomnym nomerom i,
| Element | Simvol | Atomnyi nomer |
Atomnaya massa |
Desyatichnyi logarifm rasprostranennosti |
|
| po chislu atomov | po masse | ||||
| Vodorod | H | 1 | 1.0080 | 12.00 | 12.00 |
| Gelii | He | 2 | 4.0026 | 10.93 | 11.53 |
| Uglerod | C | 6 | 12.0111 | 8.52 | 9.60 |
| Azot | N | 7 | 14.0067 | 7.96 | 9.11 |
| Kislorod | O | 8 | 15.9994 | 8.82 | 10.02 |
| Neon | Ne | 10 | 20.179 | 7.92 | 9.22 |
| Natrii | Na | 11 | 22.9898 | 6.25 | 7.61 |
| Magnii | Mg | 12 | 24.305 | 7.42 | 8.81 |
| Alyuminii | Al | 13 | 26.9815 | 6.39 | 7.78 |
| Kremnii | Si | 14 | 28.086 | 7.52 | 8.97 |
| Fosfor | P | 15 | 30.9738 | 5.52 | 7.01 |
| Sera | S | 16 | 32.06 | 7.20 | 8.71 |
| Hlor | Cl | 17 | 35.453 | 5.6 | 7.2 |
| Argon | Ag | 18 | 39.948 | 6.8 | 8.4 |
| Kal'cii | Sa | 20 | 40.08 | 6.30 | 7.90 |
| Hrom | Sg | 24 | 51.996 | 5.85 | 7.57 |
| Marganec | Mn | 25 | 54.9380 | 5.40 | 7.14 |
| Zhelezo | Fe | 26 | 55.847 | 7.60 | 9.35 |
| Nikel' | Ni | 28 | 58.71 | 6.30 | 8.07 |
| Otnositel'noe soderzhanie po masse: | Chislo nuklonov na yadro, | ||||
| Vodorod | X |
||||
| Gelii | X
|
Srednyaya atomnaya massa pri polnoi ionizacii | |||
| Prochie elementy | |||||
![]() - stepen'
- stepen' ![]() -kratnoi ionizacii
-kratnoi ionizacii ![]() -go elementa,
tak chto
-go elementa,
tak chto
![]() ,
,
![]() - massa yadra atoma s nomerom
- massa yadra atoma s nomerom ![]() i atomnoi massoi
i atomnoi massoi ![]()
![]() g - atomnaya edinica massy, ravnaya
1/12 massy izotopa
g - atomnaya edinica massy, ravnaya
1/12 massy izotopa ![]() ,
,
![]() g - massa elektrona1,
g - massa elektrona1,
|
|
(1.4) |
![]() - statisticheskii ves iona
- statisticheskii ves iona ![]() -go elementa v
-go elementa v ![]() -m
sostoyanii ionizacii,
-m
sostoyanii ionizacii,
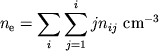 |
(1.5) |
![$$
%\begin{displaymath}\mu =\left[ \Sigma _{i}\frac{m_{u}}{m_{i}}x_{i}\Sigma ^{i}_{j=0}(1+j)y_{ij}\right] ^{-1} \end{displaymath}
\mu=\left[\sum_i {m_{\rm u}\over m_i} x_i \sum_{j=0}^i(1+j)
y_{ij} \right]^{-1}
$$](https://images.astronet.ru/pubd/2006/01/25/0001211125/tex/formula718.gif) |
(1.6) |
V polnost'yu ionizovannom gaze, sostoyashem iz vodoroda, geliya i drugih
elementov s
![]() , imeem
, imeem
![$$
%\begin{displaymath}
%\mu =\left[ 2x_{H}+\frac{3}{4}x_{He}+\frac{1}{2}x_{A}\right] ^{-1},~ %x_{A}=\Sigma _{i\geq 6}x_{i} ,~
%m_{He}\approx 4m_{u} ,~
%m_{H}\approx m_{u} \end{displaymath}
\eqalign{
&\mu\simeq\left[2x_{\rm H}+{3\over 4}x_{\rm He}+{1\over 2} x_A\right]^{-1}, \qquad
x_A=\sum\limits_{i\ge 6}x_i,
\cr
&m_{\rm He}\approx 4 m_{\rm u},
\quad m_{\rm H}\approx m_{\rm u}.
\cr}
$$](https://images.astronet.ru/pubd/2006/01/25/0001211125/tex/formula719.gif) |
(1.7) |
|
|
(1.8) |
Zdes'
![]() - energiya (potencial)
ionizacii
- energiya (potencial)
ionizacii ![]() -go elektrona,
-go elektrona, ![]() . Energii ionizacii
naibolee obil'nyh elementov privedeny v tabl. 2. Dlya nahozhdeniya stepeni
ionizacii elementov v smesi neobhodimo reshit' sistemu uravnenii (1.8)
s uchetom (1.4), (1.5). Analiticheskoe re shenie poluchaetsya v sluchae
odnokratnoi ionizacii odnogo (
. Energii ionizacii
naibolee obil'nyh elementov privedeny v tabl. 2. Dlya nahozhdeniya stepeni
ionizacii elementov v smesi neobhodimo reshit' sistemu uravnenii (1.8)
s uchetom (1.4), (1.5). Analiticheskoe re shenie poluchaetsya v sluchae
odnokratnoi ionizacii odnogo (![]() -go) sorta atomov
-go) sorta atomov
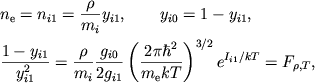
otkuda
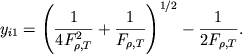 |
(1.9) |
| Atomnyi nomer | Element | Potencialy ionizacii, eV | Polnye momenty |
| 1 | H |
0.747; 13.5985 | 0; 1/2 |
| 2 | He | 24.5876; 54.418 | 0; 1/2; 0 |
| 6 | C | 11.260; 24.284; 47.89; 64.49 | 0; 1/2; 0; 1/2 |
| 7 | N | 14.534; 29.602; 47.45; 77.47 | 3/2; 0; 1/2; 0 |
| 8 | O | 13.618; 35.118; 54.94; 77.41 | 2; 3/2; 0; 1/2 |
| 10 | Ne | 21.565; 40.964; 63.46; 97.12 | 0; 3/2; 2; 3/2 |
| 11 | Na | 5.1391; 47.287; 71.64; 98.92 | 1/2; 0; 3/2; 2 |
| 12 | Mg | 7.646; 15.035; 80.15; 109.2 | 0; 1/2; 0; 3/2 |
| 13 | Al | 5.9858; 18.828; 28.448; 120 | 1/2; 0; 1/2; 0 |
| 14 | Si | 8.152; 16.346; 33.493; 45.14 | 0; 1/2; 0; 1/2 |
| 15 | P | 10.49; 19.73; 30.18; 51.47 | 3/2; 0; 1/2; 0 |
| 16 | S | 10.36; 23.33; 34.83; 47.31 | 2; 3/2; 0; 1/2 |
| 17 | Cl | 12.968; 23.81; 39.61; 53.47 | 3/2; 2; 3/2; 0 |
| 18 | Ar | 15.760; 27.63; 40.74; 59.81 | 0; 3/2; 2; 3/2 |
| 20 | Ca | 6.113; 11.872; 50.91; 67.10 | 0; 1/2; 0; 3/2 |
| 24 | Cr | 6.766; 16.50; 30.96; 49 | 3; 5/2; 0; 3/2 |
| 25 | Mn | 7.4368; 15.640; 33.67; 51.2 | 5/2; 2; 5/2; 0 |
| 26 | Fe | 7.87; 16.18; 30.65; 54.8 | 4; 9/2 4; 5/2 |
| 28 | Ni | 7.63; 18.17; 35.2; 54.9 | 4; 5/2; 4; 9/2 |
| 1 eV = 11.604 K | X |
X |
|
Pri issledovanii zvezdnoi evolyucii chasto neobhodimo znat' znacheniya adiabaticheskih pokazatelei
i teploemkostei
V usloviyah nepolnoi ionizacii vse velichiny rasschityvayutsya chislenno, dlya chego ih udobno vyrazit' cherez proizvodnye
Vospol'zuemsya izvestnymi svoistvami yakobianov
|
|
(1.10) |
Poluchaem
|
|
(1.11) |
![$$
%\begin{displaymath}\gamma _{2}=\left[ \left( \frac{\partial \ln P}{\partial \ln T}\right) _{\rho }-\left( \frac{\partial \ln P}{\partial \ln\rho }\right) _{T}\left( \frac{\partial S}{\partial \ln T}\right) _{\rho }\left/\left( \frac{\partial S}{\partial \ln\rho }\right)\right. _{T}\right] ^{-1}, \end{displaymath}
\gamma_2=\left[\left(\partial\ln P\over \partial\ln T\right)_\rho-
\left(\partial\ln P\over \partial\ln\rho \right)_T \left(\partial S\over \partial\ln T\right)_\rho \bigg/
\left(\partial S\over \partial\ln\rho\right)_T\right]^{-1},
$$](https://images.astronet.ru/pubd/2006/01/25/0001211125/tex/formula728.gif) |
(1.12) |
|
|
(1.13) |
|
|
(1.14) |
|
|
(1.15) |
|
|
(1.16) |
Proizvodnye ot entropii vyrazhayutsya cherez proizvodnye ot energii i
davleniya iz pervogo zakona termodinamiki i usloviya polnoty differenciala
svobodnoi energii ![]() :
:
|
|
(1.17) |
Esli stepeni ionizacii ![]() postoyanny, to iz (1.3)-(1.6)
slededuet
postoyanny, to iz (1.3)-(1.6)
slededuet
|
|
(1.18) |
i vse proizvodnye vychislyayutsya analiticheski:
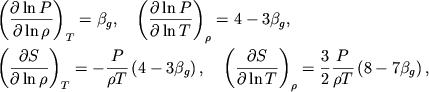 |
(1.19) |
![$$
\eqalign{
&\gamma_1=\beta_g
+{2\over 3}{\left(4-3 \beta_g\right)^2\over 8-7 \beta_g}, \quad
\gamma_2=\left[4-3\beta_g+{3\over 2}\beta_g{8-7\beta_g\over 4-3\beta_g}
\right]^{-1},\cr
&\gamma_3={2\over 3}{4-3 \beta_g\over 8-7 \beta_g},
\cr
&c_v={3\over 2}{P\over\rho T}\left(8-7 \beta_g\right),
\cr
&c_p={3\over 2}{P\over\rho T}\left(8-7 \beta_g\right)
\left[1+{2\over 3}{\left(4-3 \beta_g\right)^2\over\beta_g
\left(8-7 \beta_g\right)}\right],
\cr
&{c_p\over c_v}=1+{2\over 3}{\left(4-3 \beta_g\right)^2\over\beta_g
\left(8-7 \beta_g\right)}=\gamma_1/\beta_g.
\cr}
$$](https://images.astronet.ru/pubd/2006/01/25/0001211125/tex/formula736.gif) |
(1.20) |
Zadacha. Vyvesti uravneniya dlya koncentracii elektronov v plazme, sostoyashei
iz ![]()
![]()
![]() ,
, ![]()
![]()
![]() , a takzhe atomov i odnokratno ionizovannyh ionov
, a takzhe atomov i odnokratno ionizovannyh ionov ![]() drugih elementov.
drugih elementov.
Reshenie. Ispol'zuya formulu Saha (1.8) i tabl. 2, poluchaem dlya vodoroda
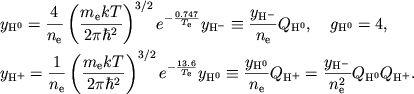 |
(1) |
Ispol'zuya uslovie
![]() , imeem
, imeem
|
|
(2) |
Analogichno dlya geliya poluchaem
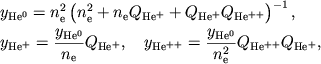 |
(3) |
gde
|
|
(4) |
i dlya tyazhelyh elementov
|
|
(5) |
Zdes' ![]() temperatura v elektronvol'tah. Ispol'zuya sootnoshenie
(4) dlya kazhdogo elementa i uslovie elektroneitral'nosti (5)
temperatura v elektronvol'tah. Ispol'zuya sootnoshenie
(4) dlya kazhdogo elementa i uslovie elektroneitral'nosti (5)
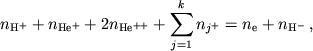
poluchaem uravnenie dlya privedennoi elektronnoi koncentracii
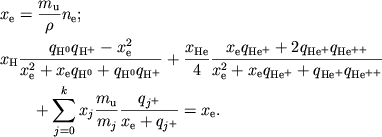 |
(6) |
Zdes'
![]() . Vse velichiny v (6) bezrazmerny
i blizki k edinice, chto udobno dlya chislennogo resheniya.
. Vse velichiny v (6) bezrazmerny
i blizki k edinice, chto udobno dlya chislennogo resheniya.
Posle nahozhdeniya stepenei ionizacii v zavisimosti ot ![]() i
i ![]() , mozhno vychislit' termodinamicheskie funkcii i ih proizvodnye.
Na ris. 1 v kachestve primera takogo rascheta privedena zavisimost'
, mozhno vychislit' termodinamicheskie funkcii i ih proizvodnye.
Na ris. 1 v kachestve primera takogo rascheta privedena zavisimost'
![]() dlya smesi s sostavom
dlya smesi s sostavom ![]() ,
,
![]() h i solnechnym sootnosheniem mezhdu drugimi elementami
(tabl. 1). Dva minimuma na krivyh
h i solnechnym sootnosheniem mezhdu drugimi elementami
(tabl. 1). Dva minimuma na krivyh
![]() sootvetstvuyut oblastyam ionizacii vodoroda i pervoi ionizacii geliya.
Pri maloi plotnosti
sootvetstvuyut oblastyam ionizacii vodoroda i pervoi ionizacii geliya.
Pri maloi plotnosti
![]() vtoroi minimum
popadaet v oblast' preobladaniya davleniya izlucheniya i potomu ne zameten.
vtoroi minimum
popadaet v oblast' preobladaniya davleniya izlucheniya i potomu ne zameten.
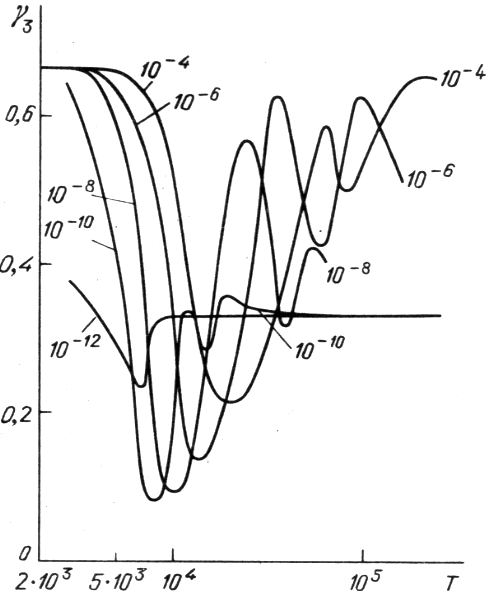 |
| Ris. 1. Zavisimosti |
<< Predislovie | Oglavlenie | 2. Relyativistskii gaz s vyrozhdeniem >>
|
Publikacii s klyuchevymi slovami:
Evolyuciya zvezd - fizicheskie processy
Publikacii so slovami: Evolyuciya zvezd - fizicheskie processy | |
Sm. takzhe:
Vse publikacii na tu zhe temu >> | |